Calomel Electrode - Construction & Working - VTU E-Learning

Calomel electrode is an example for reference electrode and an example of metal-metal salt ion electrode let us discuss the construction working of calomel electrode is.
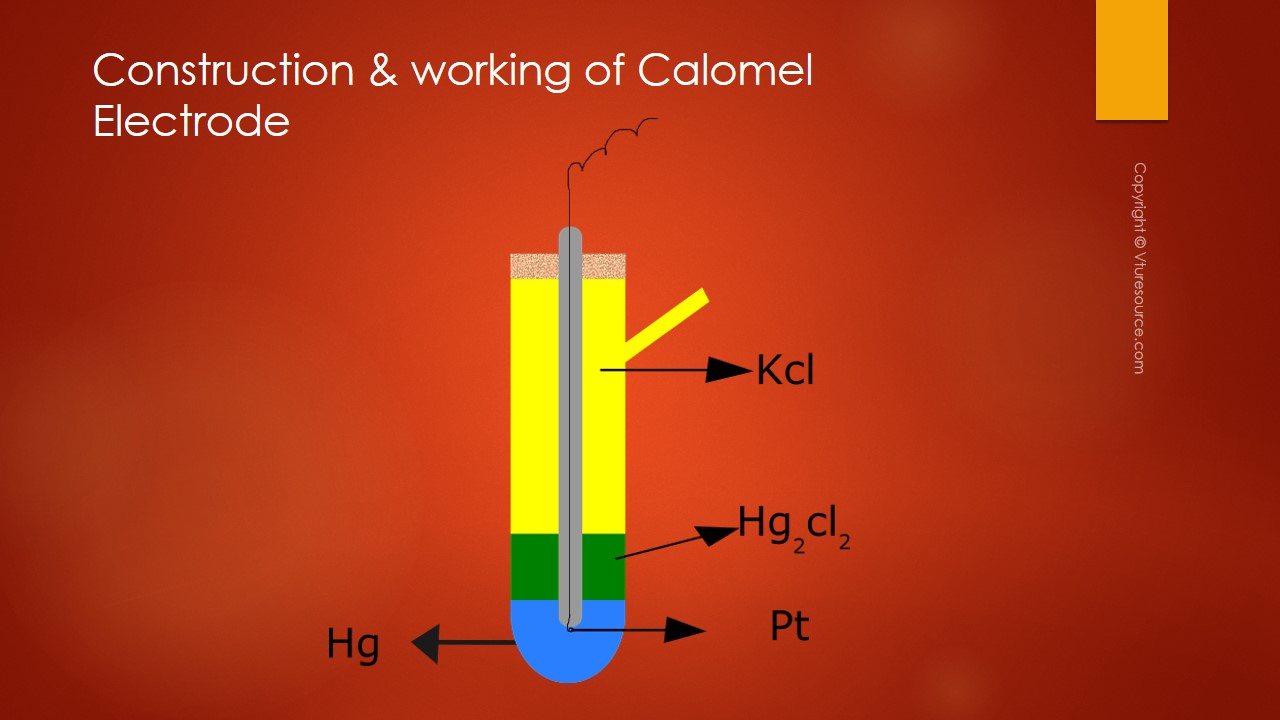
Calomel Electrode is made up of a glass tube with a side tube at the bottom of the glass tube mercury is placed above that a paste of Mercury and Mercurous chloride is placed.
Mercurous chloride is nothing but calomel so the commercial name of Mercurous Chloride is Calomel. At the bottom of the glass tube where mercury is placed a platinum metal is dipped and it is connected with a copper wire for external connection. Through this side tube, a KCl solution of known concentration is introduced.
Calomel electrode acts both as anode as well as cathode depending upon the electrode or an unknown electrode with which it is coupled with.
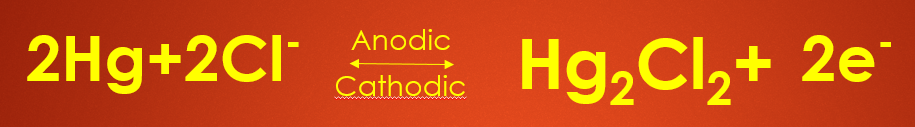
If the calomel electrode acts as an anode the anodic reaction is as follows - 2HG plus 2Cl minus will give you HG2CL2 plus 2 electrons so here mercury undergoes oxidation to liberate 2 electrons.
If calomel electrode acts as a cathode then Mercurous Chloride that is nothing but calomel HG 2 CL 2 will accept 2 electrons and undergo reduction to give you 2 HG plus 2 CL minus as all the electrodes will have an electrode potential the electrode potential of Calomel electrode is given by or determined by Nernst Equation
Which is given as E equals E naught minus 0.059 log concentration of CL - from this equation it is clearly evident that electro potential of calomel electrode at a depends upon the concentration of CL minus. For 0.1 Normal KCl electrode potential of Calomel electrode is 0.334 Volts for 1 normal KCL solution the electrode potential of Calomel is 0.28 volts and if you are using saturated KCl in the calomel electrode then the Calomel electrode potential is given as 0.2422 volts.
Categories
VTU Updates
- VTU NON-CBCS Results New
- SSP Scholarship 2023 New
- Cloud Computing vtu question papers New
- Machine Learning Syllabus New
- 18CS71-AiML VTU Question Papers New
- Machine Learning VTU Question Papers New
- Web Technology Syllabus New
- VTU change of college Procedure New
- VTU MTech Syllabus New
- VTU MBA Results New
- VTU Notes New
- VTU PhD TimeTable New
- VTU Academic Calendar 2023 Odd Sem
- VTU Updates New
- Infosys Recruitment 2022 New
- Cyber Security Syllabus New
- MBA in USA for Engineering Students New
- Contact Us